
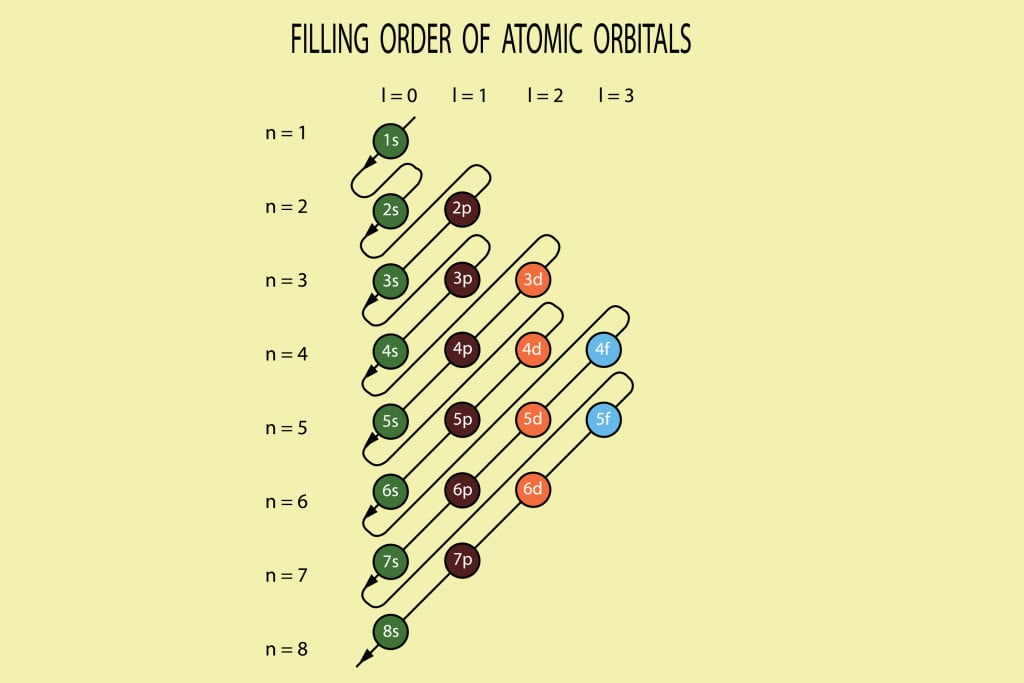
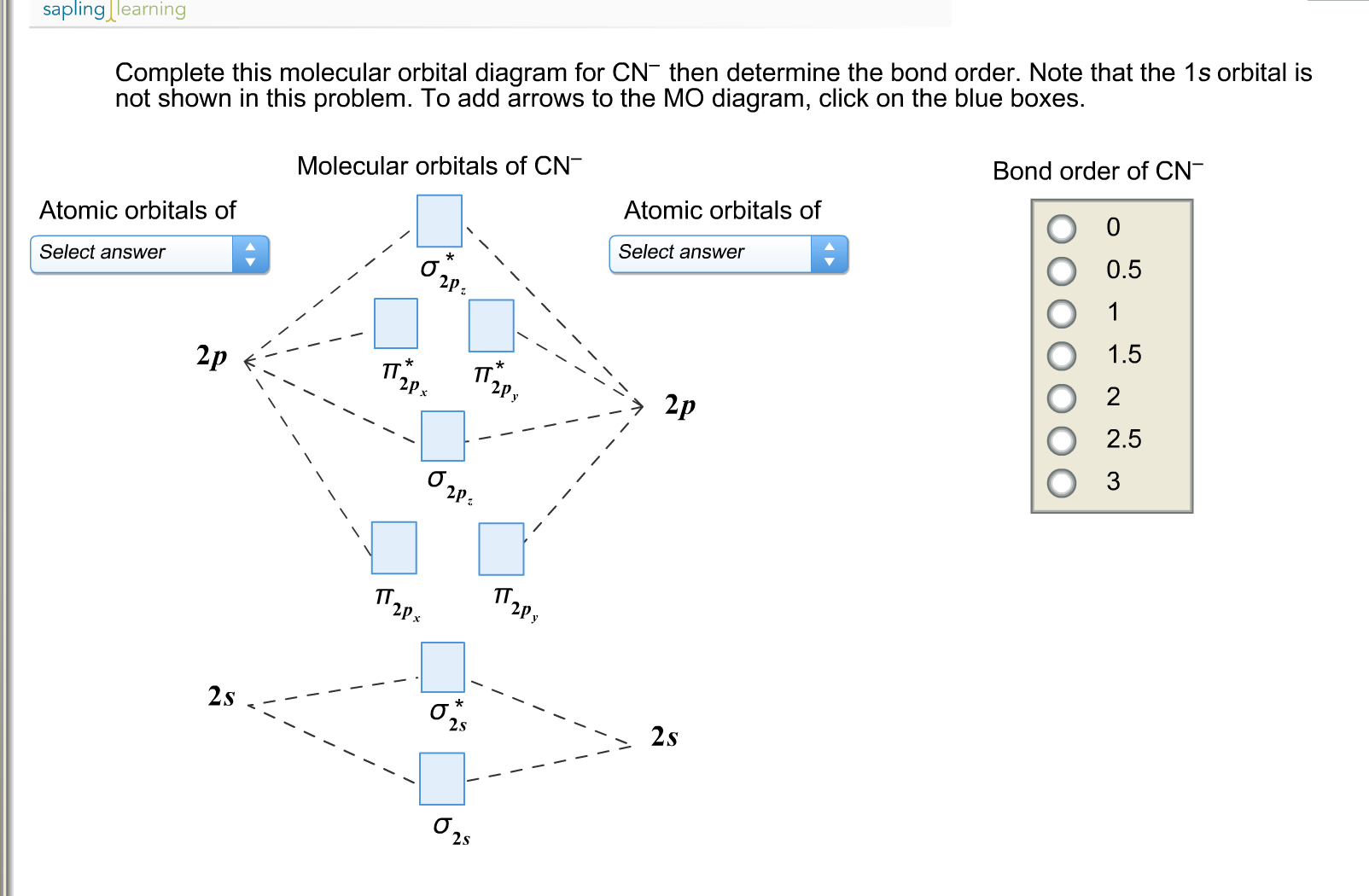
To form a triple bond, we need to have one sigma bonding MO and two pi bonding MOs. Orbitals are filled as 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 145 d 106 p 67 s 25 f 146 d 107 p 6. With two electrons in the sigma bonding MO, and two electrons in the pi bonding MO, and zero electrons in antibonding orbitals, we have an overall bond order of 1 / 2 (4 0) 2, i.e., a double bond. For example, sodium has one 3 s electron in excess of the noble gas neon (chemical symbol Ne, atomic number 10), and so its shorthand notation is 3 s 1.Įlectrons fill orbitals according to the Aufbau principle, in which the lowest energy orbitals are filled first. Often, a shorthand method is used that lists only those electrons in excess of the noble gas configuration immediately preceding the atom in the periodic table. The distance between the nucleus and the outer shell increases. In this notation, the electronic configuration of sodium would be 1 s 22 s 22 p 63 s 1, distributed in the orbitals as 2-8-1. As the value of n increases (Principal quantum number), energies of the orbitals increases. The electronic configuration of an atom in the quantum-mechanical model is stated by listing the occupied orbitals, in order of filling, with the number of electrons in each orbital indicated by superscript. SpaceNext50 Britannica presents SpaceNext50, From the race to the Moon to space stewardship, we explore a wide range of subjects that feed our curiosity about space!Įlectronic configuration, also called electronic structure or electron configuration, the arrangement of electrons in orbitals around an atomic nucleus.Learn about the major environmental problems facing our planet and what can be done about them! Saving Earth Britannica Presents Earth’s To-Do List for the 21st Century.Britannica Beyond We’ve created a new place where questions are at the center of learning.100 Women Britannica celebrates the centennial of the Nineteenth Amendment, highlighting suffragists and history-making politicians. Draw the filing order of atomic orbitals (Moeller chart) diagram when principal quantum number n 1, 2, 3, 4, 5, 6, 7, 8 and l 0, 1, 2, 3.
ORDER OF ATOMIC ORBITALS HOW TO
COVID-19 Portal While this global health crisis continues to evolve, it can be useful to look to past pandemics to better understand how to respond today.Student Portal Britannica is the ultimate student resource for key school subjects like history, government, literature, and more.From tech to household and wellness products. Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.This Time in History In these videos, find out what happened this month (or any month!) in history.#WTFact Videos In #WTFact Britannica shares some of the most bizarre facts we can find.Demystified Videos In Demystified, Britannica has all the answers to your burning questions.Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives. In quantum mechanics, atomic orbitals are described as wave functions over space, indexed by the n, l, and m quantum numbers of the orbital or by the names as.


 0 kommentar(er)
0 kommentar(er)
